Students participated in the Online EOCT CheckPoint test administered by the same corporation that makes and scores the End-of-Course-Test administered in May.
I have learned that parents do not have access to the data collected by this EOCT CheckPoint testing activity, so I will see about printing individualized reports for the parents and students.
One note, regardless of the imprudence: While this activity was a useful diagnostic tool for most of the students to assess their lack of comprehension of content material, the same small number of ignorant children who seem to perturb the learning environment on a daily basis were not only apathetic about this opportunity they were a near-continual source of disruption for the students who were working on this EOCT CheckPoint.
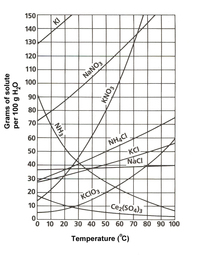
Homework was reviewed. Students were informed on how water, gases, and precipitates are clues to identifying double replacement reactions. Solubility and solution terms were reviewed, and Ionic and Net Ionic Equations were introduced.